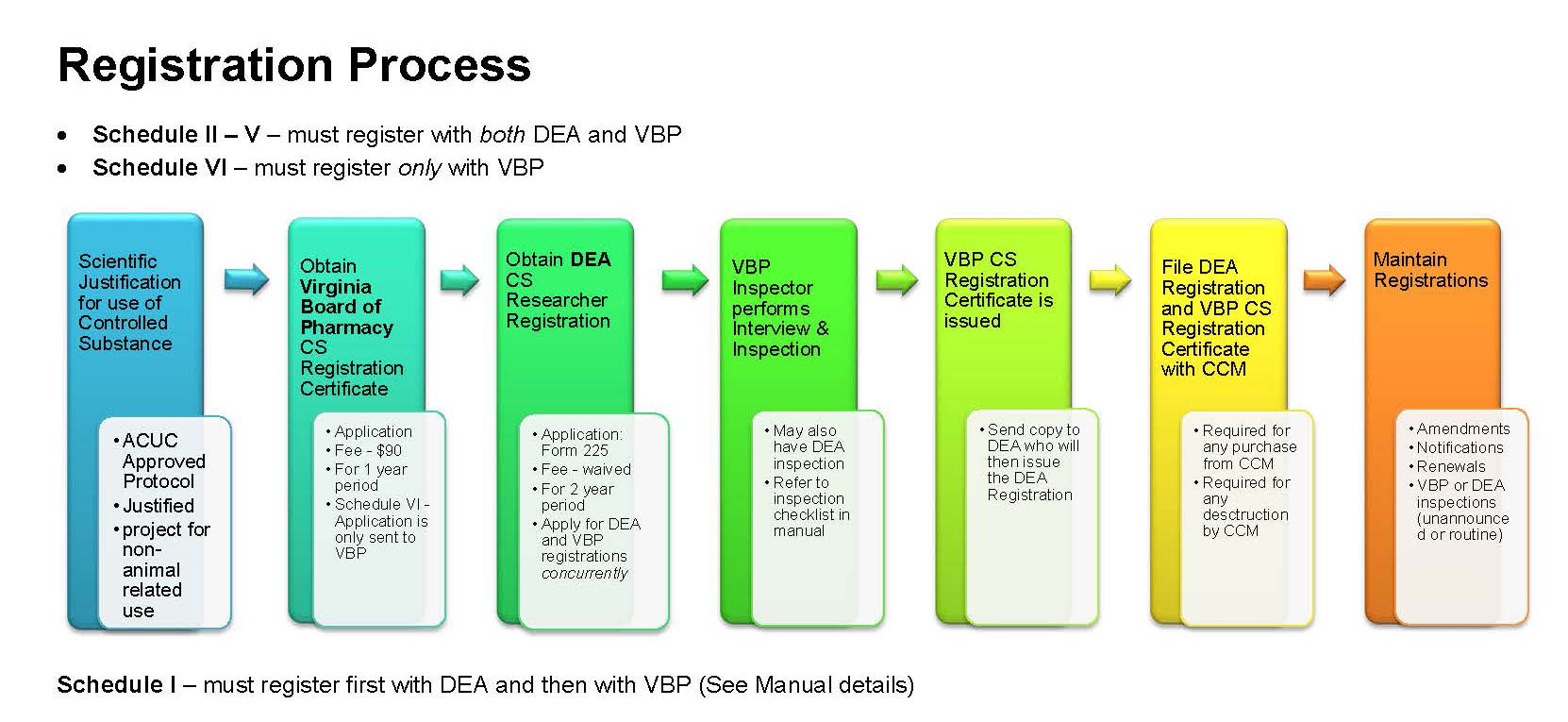
Where to Register?
Schedules I - V
Requires both a DEA Registration and VBP Controlled Substance Registration (CSR) Certificate
Schedule VI Only
VBP Controlled Substance Registration (CSR) Certificate
Obtaining a DEA Registration and VBP Registration Certificate requires an initial scheduled on-site inspection by an auditor from the VBP and possibly DEA. The VBP auditor has requested that as many inspections be scheduled together as possible for the sake of expediency.
Questions?
Feel free to contact the following people:
Angela Gamble
Director, Office of Animal Welfare
434.924.5752
Raphael Malbrue
Director, Comparative Medicine
434.924.5058
Certifying Official:
Angela Gamble, Director, Office of Animal Welfare, is the Certifying Official for DEA registration applications and renewals.
434.924.5752
VBP Registration
State Controlled Substance Application – Complete prior to DEA Application
Each Principal Investigator desiring a registration for the use of Controlled Substances in research must complete a Virginia Board of Pharmacy Application for a Controlled Substance Registration Certificate.
Request all Schedules II – VI on the same application form. The CSR application must be downloaded(see below), completed, and mailed to the VBP. Refer to the checklist located in the CS manual for guidance with assembling a complete application packet.
The cost associated with the VBP Registration cannot be waived (~$90). Pay the initial application fee with a personal check and get reimbursed through Expense UVA. Supplier/Vendor #10867. Fee can be paid using Departmental funds, non-federal funds, or personal funds. Federal grants CANNOT be used to pay registration fees.
DEA Registrations
Federal Controlled Substance Application – Complete after VBP CSR is obtained
Each Principal Investigator desiring a registration for the use of Controlled Substances in research must complete a DEA Application for Registration (Form 225) for Researcher. Request all Schedules II – V that were identified on the VBP Registration in the same DEA application. Applications must be completed online. Refer to the checklist located in the CS manual for guidance with assembling a complete application packet. The cost associated with the DEA Registration is waived when the UVA tax exempt number is provided in association with the Certifying Official.