The Institutional Review Board for Health Science Research Post Approval Monitoring and Education (IRB-HSR PAM & Ed) program serves researchers whose protocols are reviewed by the IRB-HSR.
The goals of the program are to:
- ensure the rights and well-being of research participants
- ensure the quality and integrity of the research
- identify educational needs, providing resources to meet those needs
- ensure compliance with federal, state, local and institutional regulations and guidelines
- identify areas of strength, as well as areas needing improvement, in research policies and practice
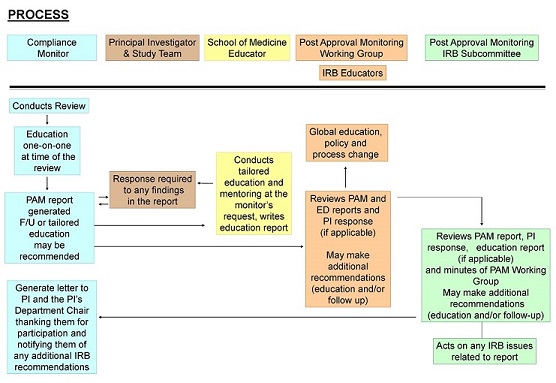
The IRB-HSR PAM & Ed program engages with IRB-HSR researchers through On-Site and Remote Review, Investigator Self-Assessment and Consent Process Observation. The review process involves the Compliance Monitor, Principal Investigator and Study Coordinators, PAM Working Group, IRB-HSR PAM Advisory Committee and the IRB-HSR. Post approval monitoring is conducted in accordance with the IRB-HSR PAM & Ed Administrative Guidance, as well as the Human Research Protection Program Standard Operating Procedures. For more information regarding the review process, see our Frequently Asked Questions.