The IRB-SBS and principal investigators are required to retain protocol records for the appropriate amount of time after a study is completed (please see Record Keeping for details). In order to maintain compliance with this requirement, the IRB-SBS needs to know when a study is completed and the protocol is no longer considered active. A protocol can be closed at any time even if the study’s aim is not met (as sometimes studies are not completed). Closing a study either means that:
- the study is permanently closed to enrollment
- all subjects have completed research-related interventions or interactions
- all long-term subject follow-up activities are complete, and
- identifiable data are no longer needed and all data has been de-identified
or
- the principal investigator no longer wishes to pursue the study.
Closing an iProtocol is only available to "approved" protocols. If your protocol has not finished the review process and you want to stop the review, you will need to withdraw the protocol.
Once an iProtocol is closed, this process cannot be undone. However, the protocol is still available and can be viewed and copied as a template for other protocol submissions. A closed iProtocol can also be reopened (see next).
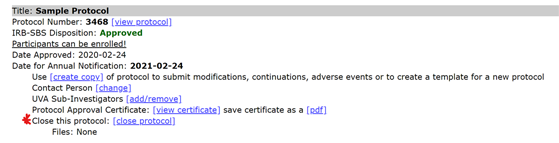
- Select the “close protocol” link below an approved protocol.
- Respond “yes” to the first question.
- Answer either “yes” or “no” regarding the reason for closing the study and provide additional information where requested.
- Select “submit.” The study is now closed.
Once an iProtocol is closed, this process cannot be undone. If the protocol needs to be reopened, it will need to be submitted for review.
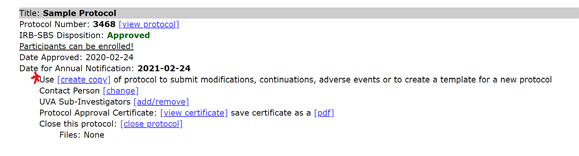
- Select “create copy” link below the closed protocol.
- Select the first radio button for a "Reopened Protocol.”
- Find the new protocol copy in the Protocol Management page and select “edit.”
- The protocol is now open and available for editing. Once edits are complete, select “submit” at the bottom of the page to submit the protocol for review.
The protocol will follow the IRB-SBS review process.
