Read-Only User access allows individuals outside of the IRB-SBS who are not otherwise associated with a protocol the ability to access basic information about the protocol and a read-only version of the protocol. Typically other offices whose business practices overlap with the IRB-SBS request this permission in order to review content that is pertinent to their office’s needs. If you think that you need read-only access to protocols in iProtocol, contact our office.
Once you are notified that you have read-only access, follow the directions below:
- Go to the iProtocol drop-down menu (horizontal bar) and select “Access iProtocol.” If you are not already logged into Netbadge, you will be asked to do so.
- On the iProtocol home page, select “Read-Only User.”
- The “Read-Only User” page provides several search options: protocol number, principal investigator (PI), Faculty Sponsor, or Protocol Title. Only one search field needs to be used at a time. Tip: you can click on the box to use it as a drop-down or you can start typing in the box to quickly access the desired protocol.
- Once you find the correct protocol number, principal investigator (PI), faculty sponsor, or protocol title, select the “select” button next to the drop-down box. If you select a PI or faculty sponsor, you will be asked to select a specific protocol number. The protocol information will appear at the bottom of the page.
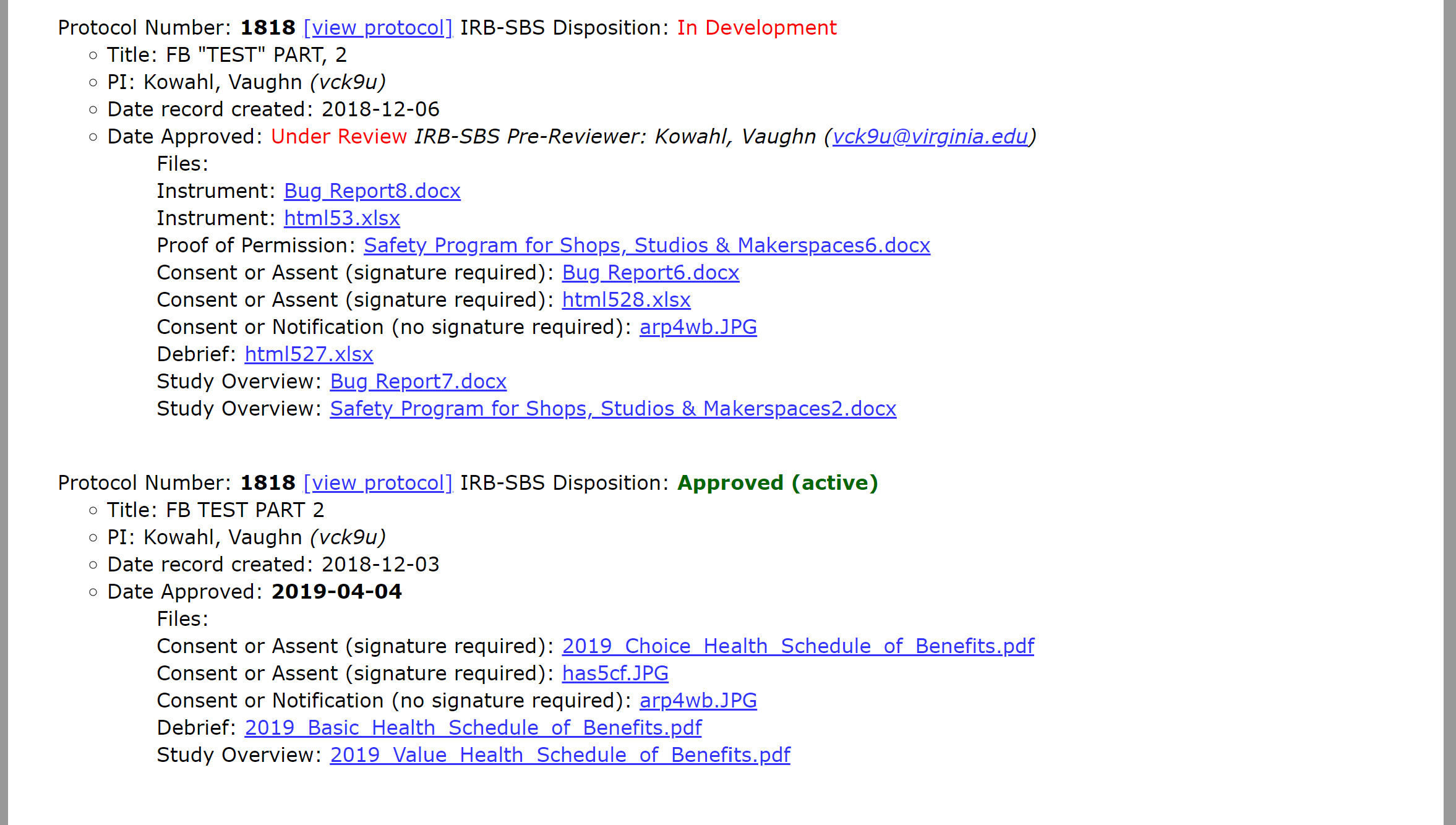
Interpreting the search results
The information provided about the protocol will give you a brief understanding of the protocol’s status in our office. In addition, you can click on “View Protocol” to read a pdf version of the protocol or click on any of the links in the “File” section to access the files uploaded with the protocol.
A protocol with this disposition is approved and actively engaged in research. The approval date is also populated with the date that our office finalized review. This version is now static and cannot be edited. Any requested edits would need to be submitted as a modification. If you have questions about this protocol, select the “email IRB-SBS” link.
The principal investigator submitted a protocol to our office and we are currently reviewing the protocol. If you have comments or revisions that you would like to request, select the “email IRB-SBS” link and send us your comments. We will incorporate your revisions into our review and make sure they are met prior to finalizing review.
If a protocol has more than one record then there is more than one version of the protocol in the system. The “approved” record is the active version of the protocol. The “in development” version is a submission that is under review—likely a modification, continuation request, or an unexpected events report (or possibly a combination of the three). Assuming the "in-development" version is approved, it will replace the "approved" version as the active version.
"Approve in Principle" is a preliminary approval for protocols that need to demonstrate to other committees that their project is approvable by the IRB-SBS even if the protocol isn't completely developed. The designation is an acknowledgement that our office has reviewed the project and found it acceptable but it doesn't represent a full approval. In order for the protocol to receive a full approval, the PI will need to make a copy of the protocol, provide a full description of the study and its involvement with human subject participants, and then submit the protocol for review.
If you saw a protocol number listed under a PI’s name or faculty sponsor’s name but no results manifested, the PI created a protocol number but has not yet submitted it to our office. You can contact the PI directly by selecting their email address and ask about the status of the protocol (or contact our office).
