In this section you will upload the documents that you will use to facilitate your consent process including materials used for recruitment, consent forms (signature required), consent notifications (signature not required), materials release forms, debriefing forms. You are not required to upload a document here; for example, if your study involves an oral consent procedure, you may not need to provide an official form (but you will want to describe your oral consent process in the “Recruitment and Consent” section).
In general, each document needs to be free from errors and needs to match what you’ve described in other sections of the protocol. The document needs to be written in a language that is sensitive to the participants' needs and written in an appropriate reading level (sixth grade reading level for most adult studies, potentially lower reading level for studies involving minors and individuals with diminished mental capacity). In short, the consent forms and other consent and recruitment documents need to communicate the elements of the study to the participants in an efficient and effective way. For more information about consent forms, see Consent Templates. The following are the various types of recruitment and consent documents:
- Recruitment Tools
- Consent Forms (signatures required)
- Consent Notifications (signatures not required)
- Materials Release Forms
- Debriefing Forms
If you haven’t developed your forms yet, templates for forms can be found on the “Recruitment and Consent Upload” page or in Consent Templates.
- Select “upload.”
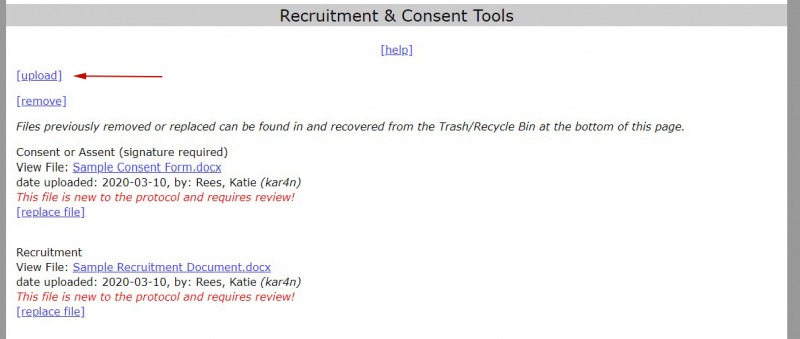
2. When you are ready to upload a document to the protocol, pick the upload category.*

3. Select “choose file” and then find the file you want to upload.
4. Select “continue” to complete the upload. The “protocol was successfully updated” page will appear to confirm the upload.
5. Select “return to protocol” to continue to edit the protocol.
To add more documents, select “upload” and repeat the steps above. For more information about managing uploads, see Creating an iProtocol.
*It’s important to select the correct category so that we can accurately document our review of this document. If you are unsure of which category to select, look at the templates lower down on the Recruitment & Consent Tools Upload page as they are organized by the upload categories.
- All consent materials are included.
- All recruitment materials are included.
- Recruitment materials that target adults state that subjects must be 18 or older to participate (or whatever age is appropriate for the age of majority if the location is outside of the US).
- The participants are informed that they will receive a copy of the consent form.
- The researcher provided a release form for publically accessible video, audio, or photographic recordings of the participants or a release form is not required for this study.
- The pre-reviewer will also determine that each consent form meets the consent form requirements that are defined in the federal regulations.
- Recruitment materials are adequate for conducting the study as described in the protocol.
- The consent forms are adequate for conducting the study as described in the protocol.
- For each consent form, the IRB-SBS Reviewer will determine the following: This consent form is appropriate for the participants and provides them with the necessary information to inform them about the study, including text provided in an appropriate reading level and native language.
