Frequently Asked Questions (FAQ)
Scroll to bottom for Tips & Tricks...
General
At go-live, ResearchUVA PBH will contain two modules: the Grants module to develop, route, and submit proposals, as well as manage awards and award modifications; and the Agreements module to create and execute negotiated funded and non-funded agreements, including outgoing subawards.
ResearchUVA PBH goes live in late-April 2022.
No, you do not need to request access. Everyone with legacy ResearchUVA access will automatically be given entry level access to ResearchUVA PBH.
This will be updated when system is live.
April 25, 2022
Please look closely at the Timeline on the About page.
April 25, 2022 is the scheduled go-live date for ResearchUVA PBH. Legacy ResearchUVA will remain available as read-only and for document download.
OSP will be providing post go-live office hours for additional support. Help can also be received by emailing ruva-huron-help@virginia.edu.
Users will have read-only access to legacy ResearchUVA. Documents will also be available for download.
A decision has not been made on this, but the system could be used for that purpose. There have been some very early discussions with research leadership on this topic. The University also will use tags to link a proposal or award with a specific intramural grant program.
Reporting
Comments and corrections are viewable in the Reviewer Notes session on a per proposal basis. There are no delivered reports on number of comments or corrections, but this could be an option for a report from the data warehouse.
Proposal Development, Review and Submission
Yes, default values can be set for Administrative Editors at the department level. When that department is selected as the Responsible Department for a proposal, those individuals will automatically have edit access to the proposal. Additional editors or readers can also be added to the proposal on a case-by-case basis if needed.
Each proposal has one “Admin Contact.” This is typically the person creating the proposal and working on it within the system. They will receive email notifications related to the proposal. The Admin Contact can be changed any time the proposal is in edit state.
Unlike today, where disclosure and training information is imported into the system, users of ResearchUVA PBH will need to go outside the system to obtain and verify such information. When we implement the Huron COI module, disclosure and related training information will be integrated into the Grants and Agreements modules.
Not explicitly. However, if a limited submission proposal is submitted without documentation of institutional approval, the Ancillary Review functionality can be used to verify and document such approval.
Non-institutional personnel that are collaborating on a proposal will need to be added for each individual proposal. Organizations you may be collaborating with (such as a Subrecipient entity or contracting party) will be added to the system for future use.
Ancillary Review functionality will be used for indirect cost waiver requests within the system. The business process for the information request and who must approve will not change substantially.
The proposal will route through the “Responsible Department” of the proposal. Ancillary reviews will need to be created for additional department approval.
I can see this getting very confusing. For example I don’t see yet where I would add a budget justification intended for submission.
This will be addressed via training. There is an area of General Submission documents on the funding proposal SmartForm and an area for budget specific attachments (such as the budget justification) within the budget SmartForms.
Data is sent to Grants.gov when the proposal specialist submits it in the SF424 module. The proposal will not be visible in Grants.gov until then.[
Budget
The federal agency forms listed below that are used for proposals submitted through Grants.gov are not currently supported for system-to-system proposal submission by ResearchUVA PBH.
- Dept of Education Budget Information for Non-Construction Programs (ED-524) V1.4
- Dept of Education Supplemental Information for SF-424 V3.0
- ED-900 General Application for EDA Programs V1.1
- ED-900B Beneficiary Information Form V1.1
- ED-900C EDA Application Supplement for Construction Programs V1.1
- EPA Form 4700-4 V5.0
- Evidence Form V2.0
- IMLS Library Program Information Form V2.0
- IMLS Supplementary Information Form V3.0
- NEH Supplementary Cover Sheet for State Councils V1.0
- Objective Work Plan V1.2
- Standardized Work Plan (SWP) V1.0
If you select an opportunity with unsupported forms, ResearchUVA PBH will provide a warning on the Funding Opportunity Announcement page. If you receive this warning, return to the Submission Information page, and update the page to indicate that you will not be submitting this application using system-to-system. See the Proposal Reference Guide for more information on system-to-system and non-system-to-system submissions. Note: the list of unsupported forms is current as of 3/28/2022. Huron maintains a current listing of unsupported forms, and the OSP Info Team has access to this list.
If your funding opportunity requires any forms on the above list, please submit using an alternative method.
If your funding opportunity includes only supported forms (and does not include forms on the above list) but you receive an error, contact the Helpdesk. The Helpdesk may be able to refresh the opportunity to allow for aa system-to-system submission.
The system has a robust budget development tool that will import salary and fringe data for UVA personnel. Multiple iterations of a budget may be created. A basic excel budget export is available in the system. For system-to-system submissions, the budget created in the system will map to the appropriate SF424 budget forms.
The system does not automatically generate budget justification language.
Clinical Trials
Both a Funding Proposal and an Agreement will need to be initiated by the study team. These can be routed simultaneously. The Agreement module will track the terms of the CTA, and the Grants module will contain the award information.
The agreements module will be integrated with DocuSign.
SF-424
Yes – The SF-424 includes numerous validations. There is a Validate action within every Smartform. For the SF-424 Smartform it will check that the SF-424 required fields are completed. The Validate Submission Activity in the SF-424 Workspace will validate in the external sponsor system. The Validate Submission Activity will lock the SF-424 to changes.
Subawards
Within the Grants module, subaward information will be part of the Funding Proposal.
Subrecipient budgets should be created as a separate budget within the Funding Proposal. Subrecipient personnel will be listed within the Funding Proposal, and Subrecipient documents, including the Consortium Commitment Form are required for the subaward package within the Funding Proposal.
After the prime award is active, to request an outgoing subaward, the study team should route an Agreement through School approvals to OSP. The Agreement module request replaces the SP-30 (Outgoing Subaward Request) form.
ResearchUVA PBH Tips & Tricks
Last updated May 26, 2022
Use Tags
When you are creating a funding proposal, you can use the “Manage Tags” activity to add tags to your proposal. These Tags identify key elements of a proposal and support reporting. Examples include tracking proposals related to internally funded seed programs, a specific program, or research area of interest. We encourage you to review the list of Tags below and add them to your proposals when applicable.
UVA Tags:
|
Grand Challenge Funding |
|
Henry Rose Carter Research Award in Malaria or Public Health (SOM) |
|
IDEAS Small/Seed Grant Program (SEHD) |
|
Ivy Biomedical Innovation Fund (SOM) |
|
LaunchPad Fund for Biomedical Innovation in Diabetes (SOM) |
|
Loaned Equipment from Sponsor |
|
COVID-19 |
|
NSF Career |
|
Pinn Scholars (SOM) |
|
SEAS Seed Funding |
|
Shared equipment/Equipment Trust Fund |
|
SOM Department and Center Funding Programs |
|
SOM Seed Funding |
|
Strategic Investment Fund |
|
The Research and Development Program (SOM) |
|
Thelma R. Swortzel Collaborative Research Award (SOM) |
|
Wallace H. Coulter Foundation Translational Research Partnership (SOM) |
|
3C Funding |
|
Annette Lightner Research Award in Rheumatology, Autoimmune Diseases, and Arthritis (SOM) |
|
Bridge Funding |
|
Course Releases |
|
Gap Funding |
Adding Someone to a Proposal
Go to the Personnel page Question 4B to enable edits rights, they will automatically have access to the award, if awarded. We, also, recommend adding your fiscal contact.
Searching in ResearchUVA PBH
There are many different locations in ResearchUVA PBH where you can search for a value such as searching for a sponsor, a person, a proposal, an award, or an agreement. By default, ResearchUVA PBH searches the system for items that begin with the value you type into a search field, but the tips below will help you improve your search.
- To expand your search, you can use the percent character (%) to search for anything that contains the value you enter.
- Search using “%Heart” when looking for a sponsor to return all sponsors that have Heart in their name (not just those that begin with Heart as you would see with the default search function).
- Search using %T32 to find a Sponsor Award ID that contains T32 within the award ID. This could be used if you are looking for T32 awards but do not know the award prefix.
- To ignore certain results in your search, start the search with “!=”
- Search for “!=SUB” on your Dashboard – My Inbox to ignore any outgoing subaward request in your inbox. This would allow you to focus on all other non-subaward project types in your Inbox.
Searching for a Sponsor in ResearchUVA PBH
The search tips listed above can be used to type into the sponsor field in a SmartForm; however, the SmartForm limits the number of search results that display. To see a list of all search results for a value, click the ellipses (…) in the righthand portion of the sponsor field to open an advance search slide-in window. The slide-in window works the same way as directly entering names into the sponsor field, but it does not limit the number of search results that display.
Adding a New Sponsor to ResearchUVA PBH (TBD Sponsors)
The OSP Info Team is responsible for adding new sponsors to the system. To alert the team of a new sponsor, select “TBD” in the sponsor field and then type in the name of the new sponsor (note that this same process should be followed for new counterparties or subrecipients in the Agreements module). The OSP Info Team will alert you when the new sponsor has been added. Note that when adding a new sponsor, the OSP Info Team completes a comprehensive process that requires compliance verification and information gathering steps.
PI Certification
PI certification is a required step for all ResearchUVA proposals – below are a few PI certification tips:
- PIs should certify their proposals prior to being reviewed by OSP or your School’s Pre-Award office (i.e., in the Draft or Department Review states).
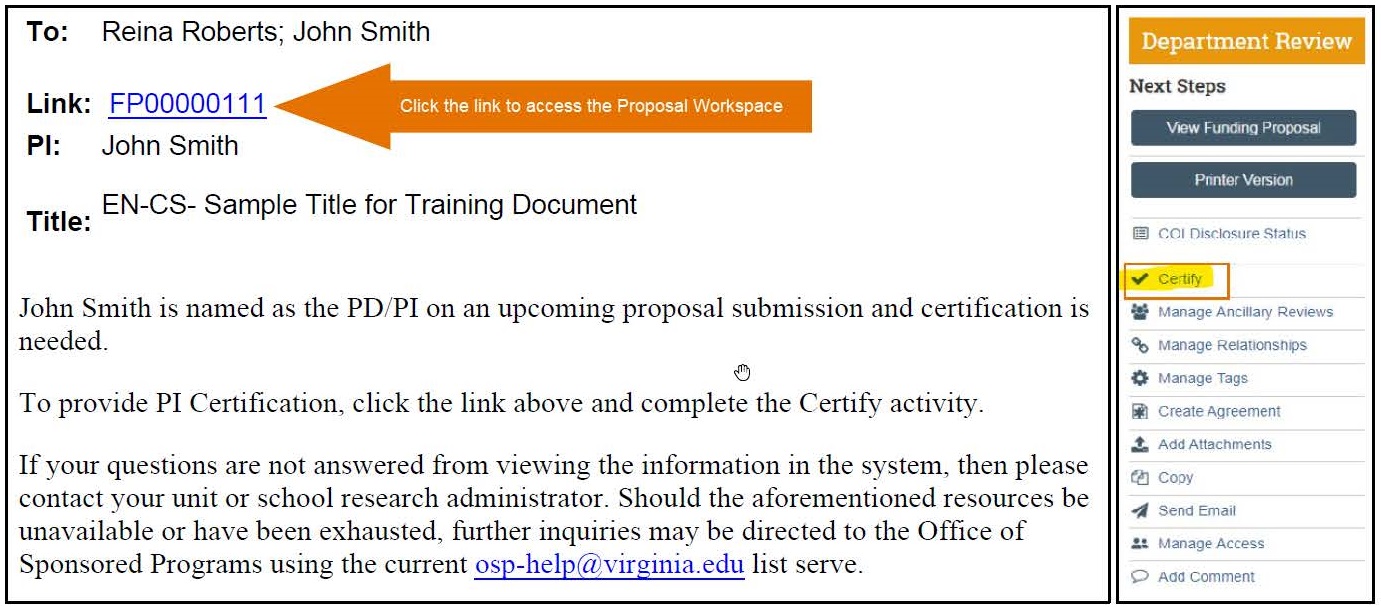
- PIs receive a notification reminding them to certify a proposal upon submission of a Draft proposal. A sample notification is included below.
- PIs certify a proposal by clicking the link in the email notification and then using the “Certify” activity on the Proposal Workspace. A sample screenshot of this portion of the Proposal Workspace is included below.
- The PI Certify step is outlined in more detail on the PI Certify Job Aid available on the ResearchUVA PBH website.
Create the .EML File
Have you ever encountered this message in ResearchUVA PBH “Note: Emails can only be uploaded as PDFs or .EML files?” There is a way around this system behavior! To create the .EML file from Outlook follow these steps:
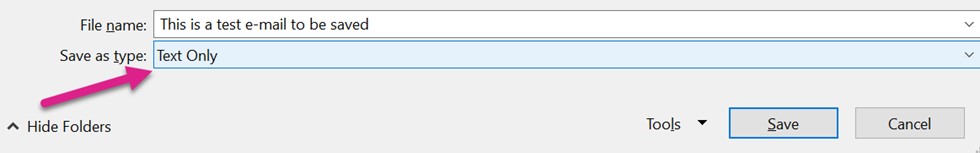
Open the e-mail and select File à Save As. Navigate to the location in which you want to save the file. Change the “Save As” type to be “Text only,” enter the name of the file, and then select “Save.”
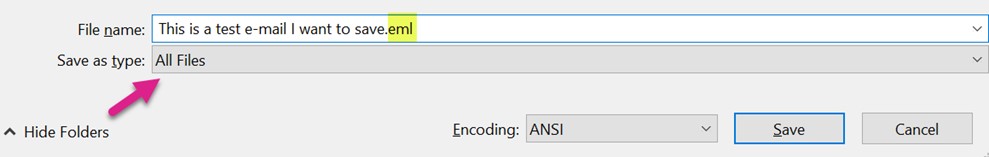
Open the Text file from your saved folder. Select File à Save As. Change “Save as type” to “All Files.” Add “.eml” to the end of the File Name. Select “Save”
Add the attachment in ResearchUVA PBH. Your e-mail is documented in ResearchUVA PBH. Note: if there are links or images saved in the e-mail, they will be lost when the e-mail is saved as a text file.
